 Michael Schramme
Michael Schramme
DrMedVet, CertEO, PhD, DipECVS
Associate Professor Equine Surgery
College of Veterinary Medicine
North Carolina State University
4700 Hillsborough Street
27606 Raleigh, North Carolina
"Recent advances in the diagnosis of foot lameness in horses"
RL:In your opinion, what are the most important advances concerning the understanding of equine foot lameness during the past few years?
MS: I think this has to be MRI and its role in identifying lesions within the horse’s foot. The list of differential diagnoses for lameness originating from the foot has substantially increased due to the improved recognition of both osseous as well as soft tissue lesions by MRI. Closely associated with this, we have also learned a lot more about the effects of local analgesic techniques used in the diagnosis of foot lameness, which is important when interpreting the clinical significance of many of those lesions seen on MRI. These two advances really have revolutionized the understanding of foot lameness in the last six or seven years.
RL: Has there been an order in the appearance of these advances…or in other words has the recognition of multiple disease processes within the horse’s foot led to re-investigation of the effects of local analgesic techniques?
MS: The recent insight into the effect of local analgesic techniques certainly predates the advances in MRI. The first paper that appeared on the effect of local analgesia of the coffin joint appeared in 1999 or 2000 in the form of an AAEP abstract. The reason why this study came about was that we observed the abolishment of lameness in a horse with a puncture wound to the middle third of the frog following analgesia of the horse’s coffin joint. As a consequence the horse was erroneously thought to have suffered a penetration of the navicular bursa even though all preceding contrast studies had been negative. Anyway, that observation triggered a discussion amongst the clinicians and at that point I recalled being taught by Professor Francis Verschooten that analgesia of the coffin joint does result in partial analgesia of the sole of the foot. And with that, the idea of investigating the effects of intra-articular analgesia of the coffin joint more closely was born
RL: Based on the improved understanding of pathological processes in the equine foot, has your understanding of navicular disease changed and more precisely do you think navicular disease is a progressive degenerative disease process which starts with mild subclinical abnormalities and progresses to cause severely debilitating tissue changes?
MS: I personally like to subdivide navicular bone disease, and I am not referring to navicular syndrome, into three separate entities. The first one is associated with lesions of the fibrocartilage, forming the palmar surface of the navicular bone as well as the dorsal surface of the deep digital flexor tendon (DDFT). In my mind this unit really behaves like a high load - low motion joint. In this disease entity, the first event is local fibrocartilage loss which then leads to cortical bone exposure and medullary remodelling as well as focal tendonitis of the DDFT. One could question which comes first, in other words what is “the chicken or the egg”: the fibrocartilage of the navicular bone or the dorsal surface of the DDFT; to me this is irrelevant as the end result remains the same.
Another navicular bone disease entity originates from the presence of distal border fragments of the navicular bone. It can be shown that these fragments do move separately from the parent bone and this seems to initiate remodelling changes at the bone interface, which I believe occurs more frequently with larger fragments in jumping horses. These focal distal border remodelling changes may eventually lead to medullary remodelling in the distal rather than the palmar aspect of the navicular bone.
The third form of navicular disease that we are able to appreciate only on MRI and subsequent histopathological examination consists of, for the lack of a better term, acute medullary necrosis. On MRI this process looks like bone oedema (very bright signal on fat suppressed sequences like the short tau inversion recover sequence (STIR)) but on histopathology this process resembles fat necrosis. Having said that, we have only seen two or three horses that fall into this category but it means that this entity occurs though probably not very commonly. Perhaps it represents some sort of acute bone contusion which then leads to focal necrosis.
RL: With respect to the focal DDFT tendonitis which you have just discussed in the context of navicular bone disease, do you think this injury predisposes the horse to go on and develop tears of the DDFT?
MS: There are different presentations of distal DDFT tendonitis. There is one form that appears concurrently with abnormalities of the navicular bone, which frequently consists of areas of superficial tendon fibrillation. Then there is DDFT tendonitis, occurring subsequent to acute traumatic tears or core lesions affecting the tendon anywhere from the pastern joint to its insertion site. Although infrequent, there can be an overlap between these two forms, as some horses may present with acute DDFT tears but show signs of navicular disease at the same time.
RL: How sensitive is MRI in identifying abnormalities of the navicular fibrocartilage?
MS: The opinions are somewhat divided with respect to the ability of MRI to identify cartilage lesions. The fact that there is a concern however, both in the human and in veterinary diagnostic imaging about the sensitivity of MRI, sends out the message that it is by no means perfect. There are two aspects to this debate: first of all fibrocartilage produces this low contrast intermediate signal that is not easily identified; so to recognize a lesion you are counting on focal bright signal reflecting the accumulation of synovial fluid in a focal cartilage defect for degeneration to become visible. Moreover, there is a normal indentation in the middle third of the navicular fibrocartilage in 50% of all horses which make the interpretation of focal synovial fluid accumulation in this location somewhat problematic.
Secondly, the thickness of the navicular fibrocartilage is at the limit of the resolution of high field MRI. In addition, the majority of the currently available MRI facilities in equine practice are standing low field magnets which provide a lower resolution when compared to that of a high field magnet. That said, of course you are not only looking at the fibrocartilage by itself but also at other concurrent signal changes at the level of the palmar cortex and medulla of the navicular bone or the dorsal surface of the DDFT which when present suggest injury to the fibrocartilage.
RL: What is your preferred sequence when imaging cartilage and what slice thickness do you use?
MS: I prefer a spoiled gradient echo, or in Siemens terminology, a 3-D, fat suppressed Flash sequence. In terms of slice thickness obviously you want to go as thin as your system will allow. Usually we collect slices of 1.5 to 2 mm thickness. We know that cartilage lesions can be very focal and if you obtain larger slices you loose the ability to detect them due to the ‘volume averaging effect’ across the thicker slice. Obviously due to time constraints and the propensity of the animal to move, the slice thickness for MRI examinations performed with the standing low field 0.3 T magnet, is usually set at 5 mm.
RL: Would you therefore prefer high field over low field MRI examinations?
MS: Really, people should not compare low with high field magnets. Each of the modalities has its specific place in diagnostic imaging. As long as everyone understands that a standing low field magnet by nature of its resolution and the slice thickness of images compromises your ability to detect subtle focal changes. That said, with the 1.5 T magnet you are able to pick up such subtle signal changes that it may become difficult to interpret these clinically. Low field MRI however has the major advantage that horses do not have to be anaesthetized for the procedure and due to much lower maintenance costs of the facility examinations can be much cheaper. In my opinion low field MRI should be a low cost / high turnover procedure which comes into play at a much earlier stage of a horse’s lameness investigation and could precede a high field MRI examination if the results turn out to be negative or equivocal. Unfortunately it seems that a lot of the standing low field MRI facilities have set their charges in a similar price range as the high field centres. If the cost to the client was truly similar for both modalities, I would have no hesitation to advise owners to go for a high field 1.5 T examination, since the anaesthetic risk in horses can be kept at an absolute minimum nowadays. I can not recall a single anaesthetic accident in the 150 – 200 MRI examinations so far performed under general anaesthesia here at North Carolina State University .
RL: Do you think MRI examination should become part of a pre-purchase examination now that MRI has become such a widespread, readily available modality?
MS: Yes, I think it could be part of a pre-purchase examination, especially if you are interested in buying a valuable horse. For example thinking of chronic collateral ligament or tendon injuries which are invariably difficult to detect clinically, I think those are things that you would like to know prior to purchase. I would like to emphasize however that radiography and MRI are complementary to each other and that MRI should certainly not replace radiography. For example periosteal new bone or osteophyte formation is difficult to detect or paradoxically easy to over-read on MRI due to slice thickness but on radiographic images it is more clearly visible. So, what I do not like and it seems to be getting more frequent, is that horses are presented for MRI examination but have not had a proper radiographic examination performed, because there is considerable danger of missing or misreading MR changes without knowledge of the radiographic appearance of the area.
RL: How much does the magic angle artefact affect your examination of the horse’s foot.
MS: Well, the magic angle artefact can not be avoided and it is something you have to work with and of course it becomes an important issue with respect to the examination of the distal portion of the DDFT. Short echo time sequences such as T1 and proton density sequences are more susceptible to the magic angle artefact. Therefore T2 and STIR sequences are an essential part of the examination to avoid missing lesions in the area of the magic angle artefact. The collateral ligaments of the coffin joint and the oblique distal sesamoidean ligaments are also dangerously close to the magic angle and there is more and more evidence available that some hyperintensities may be due to that effect rather than an injury per se. In addition we need to remember that ligaments are not as uniform in their fibre orientation as tendons and this may give raise to “patchy” areas of hyperintensity or lack of homogeneity within the ligament. And again certain sequences are more susceptible to this effect, such as the T2* sequence when compared to spin echo sequences in low field magnets. In order to avoid that problem especially when working with low field systems you have to make sure that spin echo sequences are included in the MRI protocol in order to assess the collateral ligaments accurately.
RL: Based on your experience and the knowledge of common MRI findings, what is the most likely lesion in horses in which lameness is abolished by a palmar digital nerve block?
MS: Without a shadow of a doubt the most likely lesions are degenerative changes at the interface between the navicular bone and the DDFT. Certainly from our histological surveys and also from Ian Wright’s work these are the most common pathological changes found in horses with palmar foot pain or “navicular syndrome”.
RL: Do you think there is significant clinical information to be gained from the shape of the navicular bone or the number of its synovial invaginations?
MS: That’s hard to say. Prof. Kees Dik came up with this composite grading system for navicular bones in which the shape and number of synovial invaginations play a certain role. Since this grading system has been applied for breed selection purposes in Holland, the incidence of high grade navicular bones (grades 3 and 4 out of 4) in the Dutch Warmblood population has dramatically reduced, suggesting that the grading system has its merits. Personally I have doubts on the importance of the shape, but above all Kees Dik’s grading scale certainly has facilitated communication between veterinarians. One thing I disagree with in Dik’s work is his grading of distal border fragments as more conditionally acceptable findings while I would see them as a reason to fail a horse.
RL: Finally in respect of the case with navicular disease presented in the case study section of the website, does it surprise you that the less lame leg contained the more severe histopathological changes?
MS: No, not at all. There is very poor correlation between radiographic changes and clinical disease, similar to what we can experience with osteoarthritis of the distal tarsal joints for example. In addition, the discrepancy between lameness and the disease status of the navicular bone may be explained by the presence of more substantial soft tissue injury in the leg with the milder bone pathology. Obviously MRI would have been the diagnostic modality to elucidate this aspect, although radiography was sufficient for the diagnosis.
RL: Can you speculate on the abnormalities that MRI would have shown in this horse’s feet?
MS: In advanced cases like this you would have seen medullary remodelling consisting of increased fluid signal on fat suppressed images and concurrent sclerosis. I would also guess that you would have seen changes at the dorsal surface of the DDFT accompanying the erosive changes of the palmar surface of the navicular bone, which all together leads to an inflammatory response in the navicular bursa, thickening of the bursal lining, the impar ligament and the navicular suspensory ligament, and possible adhesion formation between the DDFT and the flexor surface of the navicular bone and the DDFT and the navicular suspensory ligament. (Figure 1, 2 and 3)
RL: What is your preferred treatment in a case that blocks to intrabursal analgesia of the navicular bursa.
MS: To make an accurate decision one should find out what the MRI findings are since a lesion of the DDFT (core lesion or longitudinal tear) is not a suitable candidate for intrabursal corticosteroid treatment. Neither would I use vasoactive medications such as isoxsuprine combined with sustained exercise with or without phenylbutazone in a horse with a DDFT injury, although they are obviously suitable for a horse with navicular bone disease.
Depending on the MRI findings we may be dealing with one of three possible scenarios in horses that block sound to analgesia of the navicular bursa; (1) a horse with fibrocartilage erosion, superficial fibrillation of the DDFT and navicular bursitis, or (2) a horse with a primary DDFT injury or (3) a horse showing both of the above which tends to be the most difficult type of case to manage. In the first instance, systemic NSAIDs and corrective farriery would be indicated followed by intrabursal medication using triamcinolone acetonide and hyaluronic acid. Unfortunately once intrabursal corticosteroid administration is initiated, you tend to be on the slippery slope of repeat medications and as we all know the effect of repeat medications becomes less satisfactory each time. Commonly this line of treatments allows for successful management for a duration of 2-3 years. Ultimately however when these treatments become ineffective palmar digital neurectomy must be considered.
In horses with DDFT lesions, six months of stall rest has been shown to be successful in about 30 percent of the cases which of course is disappointing. One thing that has been evaluated is the use of bursoscopy. Areas of torn tendon fibres within the synovial compartment of the navicular bursa have the tendency to heal poorly since natural debridement is impaired in the synovial environment. Therefore the theory behind surgical debridement of torn collagen fibres in a synovial compartment is that it triggers an intrinsic healing process preventing continuation of bursal inflammation. In a study of 20 horses with DDFT lesions that communicated with the navicular bursa, bursoscopic debridement of torn tendon fibres allowed 11 out of 15 horses for which follow up information was available, to return to their previous use {Smith et al., 2007 }.
Recently we have started to perform intralesional or intrabursal injection of bone marrow-derived mesenchymal stem cells and so far, of six treated horses 4 have returned to exercise. These are of course small numbers, but the result suggests that this may be an valuable alternative treatment modality. Currently I believe DDFT lesions which are in communication with the navicular bursa are best treated with bursoscopic debridement and injection of stem cells three to four weeks later.
RL: With respect to bursoscopy being a diagnostic and therapeutic tool, should horses undergo this surgical procedure routinely once they respond to intrabursal analgesia of the navicular bursa?
MS: In the study by Smith, every horse that blocked to the navicular bursa had pathology in the bursa with 95 percent of pathology involving the DDFT. I think in an area lacking an MRI facility, this would certainly be a valid way forward, especially as it allows both diagnosis and therapeutic intervention at the same time.
RL: Thank you for your time.
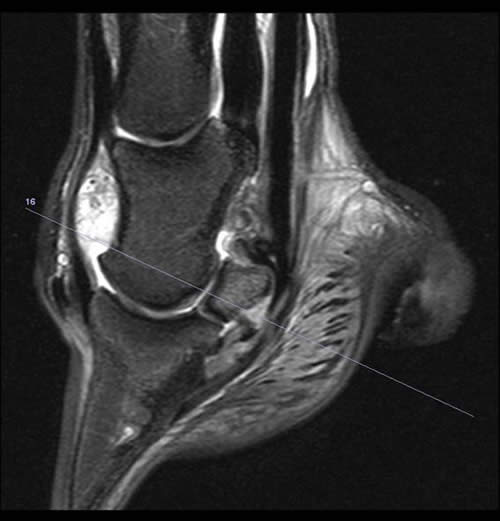
Figure 1: Sagittal 2D STIR sequence showing hyperintense signal at the palmar aspect of the navicular bone and in the DDFT consistent with fibrocartilage loss, cortical demineralisation, focal oedema and fibrosis of the medullary cavity and focal tendonitis of the DDFT (long arrow). In addition the navicular bursal space is obliterated by soft tissue swelling of the impar ligament and adhesion formation between the DDFT and adjacent structures (short arrow).
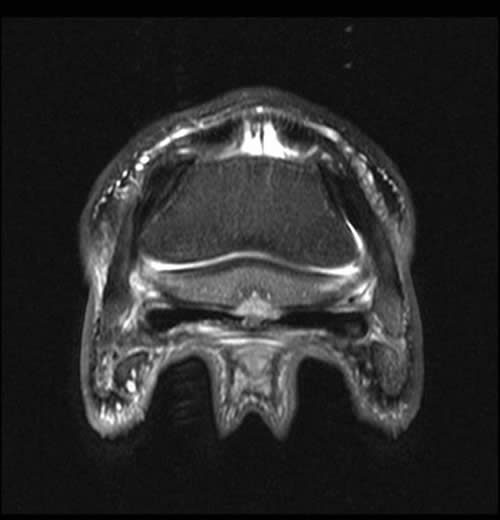
Figure 2: Transverse 2D STIR image of the same horse as shown in figure 1. There is focal degeneration of the DDFT and focal palmar cortical lysis (arrow). Adhesion formation between the palmar cortex and DDFT is likely and there is mild medullary oedema of the navicular bone.
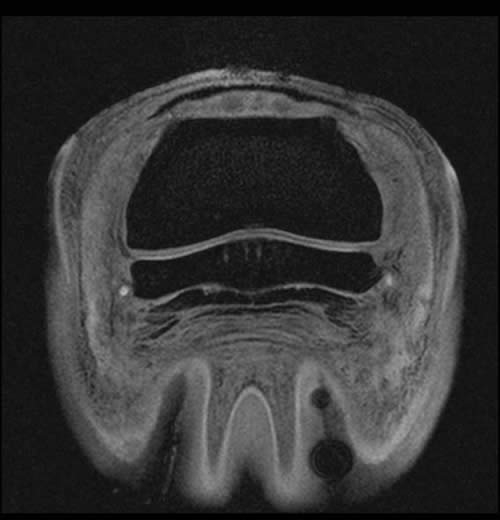
Figure 3: Tranverse 3D Flash MRI sequence with fat suppression of a horse’s foot; focal dorsal fibrillations of the DDFT can be appreciated (white arrows). Concurrent areas of fibrocartilage loss from the palmar surface of the navicular bone are certainly present but harder to recognize.















