 Dr. Denis Marcellin-Little
Dr. Denis Marcellin-Little
Associate Professor, Orthopedics
Department of Clinical Sciences
College of Veterinary Medicine
North Carolina State University
Dr. Marcellin completed his doctor of veterinary medicine training in Toulouse, France. He is a Diplomate of both the European and American College of Veterinary Surgeons. Dr. Marcellin is recognized as an authority in the field of veterinary orthopedics. His areas of interest include total hip replacement, external fixation, treatment of bone deformities and physical therapy. His current research interests include the biological response to orthopedic implants, distraction osteogenesis and canine developmental orthopedic diseases.
In a recent publication in the journal Veterinary Surgery1, Dr. William Liska and Dr. Marcellin-Little and others describe the development and implantation of a custom total knee prosthesis in a dog following a traumatic fracture and non-union. This is the first such description of the use of biomodeling techniques to develop a custom orthopedic implant for the treatment of a complicated injury in the veterinary literature. I would like to discuss with Dr. Marcellin-Little, his experience with biomodeling and how this technology can be applied to both research and clinical orthopedic cases.
Dr. Marcellin, thank you for taking the time to speak with me.
Q: How did you become interested in custom implant development?
A: I’ve always been interested in challenging cases. In referral practice, we are often presented with cases that are puzzling, those for which there is no classic solution for an orthopedic problem. Those patients are natural cases for the development of new methods. Initially, I had a handful of complex cases that created challenges. The development of new methods comes from being challenged by cases that are not readily treated by conventional methods. The most challenging cases would be those that involve complex deformities or abnormal joints. These are the ideal candidates for modeling or custom implant development.
Q: What are the steps involved in the development of a custom prosthesis or model?
A: The foundation is based on a CT scan. There has been great progress over the last 5 years made in improving our CT scans. They are now faster and more precise. After the scan is complete, on the engineering side we use software to translate the CT scan data to a computer aided design (CAD) file. There are several different options available for doing this. The most widely used is a program called Mimics®. The CAD file is then fed into one of several machines that can make the replica or model. This is known as rapid prototyping (RP). There are a lot of different techniques in RP. Some machines make the [model] directly in a polymer such as ABS plastic or polyurethane or wax impregnated plaster. Sometimes we can make a cast or mold either directly (hollow mold) or using a technique called room temperature vulcanization to create a silicone mold based on the original. This can be used to replicate a model multiple times using other materials that can be injected or cast into the mold. At NC State our models are made using a process known as stereolithography or SLA. The CAD file is fed into a machine, which then uses a laser to solidify liquid plastic poured in subsequent layers to build up the final model. Custom implants can be made from metal. We have the great luxury of having this capability at North Carolina State University. There are several different techniques to do this, here we use a process called electron beam melting (EBM) to create custom implants using various metals.
Some materials are more durable than others. We can also create models of different sizes depending on the need (for demonstration or rehearsal) or the added cost to the client. At this point we are still making replicas that are identical to the original bone, mainly to be used for diagnostics and the design and rehearsal of a surgical procedure.
Q: How much additional cost does the development of a biomodel or custom prosthesis add to the patients’ bill?
A: A CT scan is the first expense and in these complex cases is generally needed for diagnostic purposes. Some patients already have had a CT scan done during their initial workup. Beyond this, there is a fee for the design and production of the model. This can be as little as $100 or as much as $1000 for a very large model. On average, the cost is $200-300 for a single model. We often will make 2. One is kept as a reference and one is used for design and rehearsal of the surgery. With custom implants or prostheses, the cost is greater as metal always cost more to produce.
The design costs are variable. In the human field a custom implant may cost as much as $30,000. There is obviously a lot of time and expertise involved in engineering, manufacturing and finishing of implants. The machines are very costly as are the software, training and design involved. We benefit from our non-profit nature, and as a result, our implants end up costing a few thousand dollars depending on the complexity. My purpose is not to make money, but rather to streamline the design process. As this process becomes even more streamlined, the cost will become lower.
Q: How long does it take to create a model once the CT scan is done?
A: Five years ago, the turnaround time used to be several month, now we can have a model within a few days if necessary. Generally, it takes around a week without problems.
Q: As justification for the additional cost to the client, what are the benefits to creating a custom model or implant– an increased surgical experience? Decreased complication rate? Shorter surgery times? Improved outcome? Treatment of conditions formerly thought of as untreatable or prone to failure?
A: All of the above. I try to be pragmatic and always use simplest or least expensive method of management. This, however, is not always possible. We have been designing procedures that did not really exist in the past, for example, the increasing complexity of our hinged ring fixators.
Another benefit is being able to design implants that have a predictable fit. In the case of the custom total knee replacement1, the [model] helped in the development of the prosthesis which had a predictable fit. I think we get a better understanding of problems by having a replica of the bone to work with.
Q: It is obvious that the creation of custom implants or a biomodel requires the involvement of those outside of the medical field such as engineers and material scientists. What is the general approach to this process and how can other surgeons integrate this technology into their practice?
A: It is a constant exchange, a team effort. In this, it is like rehabilitation. The expertise of a single clinician is rarely enough to develop a good treatment plan. I feel fortunate that we have a good nucleus here at NC State that has allowed us to expand our biomodeling applications widely over the last 5 years. It would be impossible for us to have done that without our other team members and vice versa. You need members of the team that are comfortable working with CAD, free-form fabrication and the orthopedic problem at hand. Alone, we could not go very far. The success of the team hinges on every one being pretty good at what they do.
For another clinician that is interested in starting to use biomodeling, they can send a CT scan, made specifically for the purpose to an outside party to have a model or implant created. In this way, the clinician doesn’t have to be an expert in biomodeling to benefit from the technology. In the future, modeling will be used much more routinely for complex cases. Even if the machines that are needed are not as readily available, there will be centers that provide this service for clinicians.
Q: This technology has obvious benefits with regards to research and surgical training, allowing the creation of precise and reproducable anatomic models. In a 2005 publication in the American Journal of Veterinary Research2, you reported the results of in vitro testing of five canine tibial plateau leveling methods. As a component of this research, you utilized biomodeling and rapid prototyping techniques to develop and test different surgical procedures using identical canine tibia models. Do you feel as though this technology will allow for more reliable research results?
A: Yes. I was actually a bit disappointed with the models in that study. Even though they did what they were designed to do, there were significant challenges in their design. The way we cast them, to make them as natural as possible created some challenges. There was a fair to higher variability than we would have liked with those models. We are getting better at the process though.
I think it would be better to be using models than natural samples in research whenever possible. It is getting more difficult to collect samples from live or deceased patients and there is some appeal to be creating your own samples. You can build them with the features that make them readily testable which has the potential to enhance the precision of the testing, such as those in the plateau leveling study. In the future, there will be an increased availability of the technology, and these models can be customized and more reproducible. This seems like an attractive product for biomechanical research. Because the files are in CAD format, the transition to finite element analysis (FEA), which uses the data within CAD files for testing, is much easier. Ultimately, in the future, mechanical testing will become less necessary and virtual testing using FEA should become more universal. Because of the current challenges with the complexity of the problems we face, we hope that as computing power and experience improves, we will able to do our testing virtually.
On a more clinical side, I hope that we can also begin to use more virtual technology. Building models is a good start, but I hope that we can design and rehearse surgeries virtually. This will decrease the cost to the client as well. There are several pieces of software that are aimed at doing virtual surgical rehearsal, though we are still quite a way from that at this point.
Q: What other applications exist for this technology beyond treating deformities and designing custom implants?
A: There is a natural extension of modeling into any complex situation, where a clinician is speculating about a problem or exploring an abnormal region before deciding what to do. The concept of performing an exploratory surgery is suboptimal in management. The clinician is forced to perform the double duties of analyzing and treating. The modeling or reproduction of an abnormal region can function as a diagnostic tool. This has logical applications for neurosurgery because of the complexity of the spine and similarly for surgery of the head. The thoracic and abdominal organs like the heart and liver can be further evaluated using models as well. This can include rehearsal of complex or unique surgical procedures. Any situation that is complex and life threatening could potentially be assessed before surgery.
Q: How can biomodeling be applied to education or teaching?
A: Rapid prototyping was initially used for this purpose. The techniques were first used by human anatomists. They would reproduce anomalies and exchange them for the purpose of teaching clinicians. It is obviously a better way to teach what can go wrong. It also has great value for the owners. Having a replica on their hands can help them to appreciate the severity or complexity of a problem. It can also help the clinician to explain the details of the surgery that is planned.
For the clinician, it can allow them or other clinicians being trained, the opportunity to perform the surgery on the model prior to performing it on the patient. As long as the owner or clinician can manage the costs, then there is a clear benefit.
Q: How have you used these rapid prototyping and biomodeling techniques in the clinical situation?
A: We started with limb deformities. Initially we used biomodeling for diagnostic purposes in patients with complex multifocal deformities, particularly those of the pelvic limb, which are often more puzzling than those of the forelimb. We then transitioned to using our models for surgical rehearsal in these patients and also those that were too small, at the limit of a certain technique or those with multiple problems in a single bone or adjacent bones. After this we started to utilize the techniqes to plan and rehearse surgeries involving osseointegration. We are currently working with limb-sparing techniques using biomodeling, the design of custom bone plates and implants as well as the design of external prostheses or prosthetic extremities and protective braces for joints.
We anticipate success much more than we used to 20 years ago, when we were trying new surgeries for the first time. Now, the cost of surgery is greater and owners expect success more than they used to. Having the option to rehearse a surgery makes the results more predictable. Even with that, not every angular deformity, for example, requires a model. I am primarily using biomodeling for unique or uncharted deformities and in those patients where specific features of their bone (the shape, size, origin of the deformity or specific malformations) would make a treatment potentially risky or unpredictable.
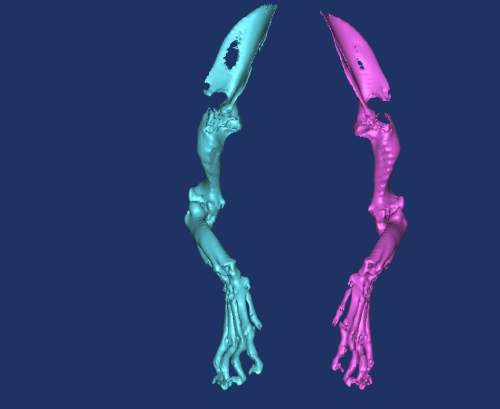
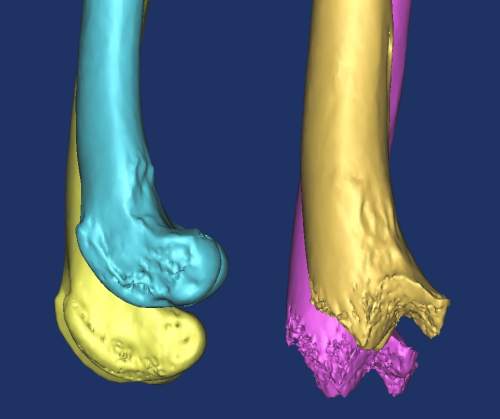
Q: What other areas of surgery would benefit from such technology?
The techniques have applications for those patients in need of custom total joint prosthesis, such as that case reported by Liptak et al.3 [a study in which a custom total hip prosthesis was used to treat a dog with a proximal femoral osteosarcoma]. We are basically developing specific implants for patients with unusual or severe problems. This would include loss of bone due to trauma and excision of bone because of neoplasia. Look to the human field as well. There is room for custom implants in people, and a similar need in our patients. Limb-sparing surgery is an area to develop significantly. Some of our patients are not candidates for amputations. Limb sparing with custom prostheses is natural in people and it should be in animals as well. Having the option to replace large portions of bones or entire joints would be of great benefit. I also see a great benefit of this technology for neurosurgery, surgery of the head (including intraoral, intranasal and maxillofacial surgery) and the design of osseointegrated implants as well. We will have the ability to design implants that are more sophisticated and adapted to our patient. This can also be used to evaluate developmental anomalies. In human medicine, rapid prototyping has been used to rehearse the separation of conjoined twins. The ability to study complex vascular anatomy would be a benefit to the soft tissue surgeon. We are lagging behind human field, though I am hopeful that we will be catching up in the future.
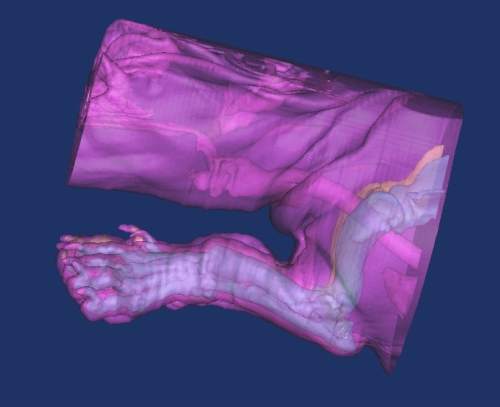
Q: In your most recent publication in Veterinary Surgery1, you report the results of the development and implantation of a custom total knee replacement prosthesis for a dog with a fracture and non-union of the distal femur. This patient likely posed some unique challenges because of the complex nature of this injury. What problems did you recognize and overcome in the treatment of this injury?
A: Though each problem poses a new set of challenges, we always come back to the basic principles of orthopedics. There are only so many ways to fix metal to bone. The problems we face are ultimately universal. Custom implants are similar to a conventional total hip prosthesis and the universal rules still apply.
Q: In human medicine, rapid prototyping techniques have been utilized for tissue engineering – producing patient specific biological substitutes to help replace damaged tissues or organs. One specific area of interest to the veterinary orthopedic surgeon is the development of custom biocompatible or bioactive implants to treat segmental bone defects. Specifically, for what aspects of veterinary orthopedics would this be most useful?
A: We are working on this, with cell seeding of implants. We’ve made 3D meshes with cell seeding and some research is ongoing. Obviously cell cultures add to the cost and complexity of an implant. There is less direct clinical benefit at this stage. The research into this area of technology is more fundamental. Looking at combining a form with organ growth for the management of cancer or chronic organ failure is a longer term application. I am currently interested in reabsorbable 3D scaffolds and custom implants. We have several graduate students working on this as we speak.
References:
- Liska WD, Marcellin-Little DJ, Eskelinen EV, Sidebotham CG, Harrysson OL, Hielm-Björkman AK. Custom total knee replacement in a dog with femoral condylar bone loss. Vet Surg. 2007 Jun;36(4):293-301.
- Hildreth BE, Marcellin-Little DJ, Roe SC, Harrysson OL. In vitro evaluation of five canine tibial plateau leveling methods. Am J Vet Res. 2006 Apr;67(4):693-700.
- Liptak JM, Pluhar GE, Dernell WS, Withrow SJ. Limb-sparing surgery in a dog with osteosarcoma of the proximal femur. Vet Surg. 2005 Jan-Feb;34(1):71-7.
All images courtesy of Dr. Denis Marcellin-Little















